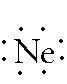
Examples:
Lithium:
1s2 2s1 -- 2 is the
outer energy level. Since it is 2s1, there
is only 1 electron in the outer energy level. Thus the Lewis Dot Diagram
should look like:
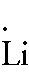
Beryllium:
1s2
2s2 -- 2 is the outer energy level. Since it
is 2s2, there are 2 electrons in the outer
energy level. Remember, both of these electrons in the outer energy
level are in the s-orbital. Thus they should be written together and
the Lewis Dot Diagram should look like:
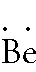
Boron:
1s2 2s2 2p1
-- 2 is the outer energy level. Since it is 2s2
and 2p1, there are 3 electrons in the outer
energy level. Remember, both of the electrons in the s-orbital have
to be written together. The 1 p-orbital electron can go in any empty
space. The Lewis Dot Diagram should look like:
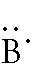
Carbon:
1s2 2s2 2p2
-- 2 is the outer energy level. Since it is 2s2
and 2p2, there are 4 electrons in the outer
energy level. Remember, both of the electrons in the s-orbital have
to be written together. The 2 p-orbital electrons will each fill an
empty space before they pair up. The Lewis Dot Diagram should look like:

Nitrogen:
1s2 2s2 2p3
-- -- 2 is the outer energy level. Since it is 2s2
and 2p3, there are 5 electrons in the outer
energy level. Remember, both of the electrons in the s-orbital have
to be written together. The 3 p-orbital electrons will each fill an
empty space before they pair up. The Lewis Dot Diagram should look like:
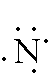
Oxygen:
1s2 2s2
2p4 -- 2 is the outer energy level.
Since it is 2s2 and 2p4,
there are 6 electrons in the outer energy level. Remember, both of the
electrons in the s-orbital have to be written together. The 4 p-orbital
electrons will each fill an empty space before they pair up. Since there
are only 3 empty spaces but 4 p electrons, two of the electrons must
double up. The Lewis Dot Diagram should look like:

Fluorine: 1s2 2s2
2p5 -- 2 is the outer energy level. Since it
is 2s2 and 2p5, there
are 7 electrons in the outer energy level. Remember, both of the electrons
in the s-orbital have to be written together. The 5 p-orbital electrons
will each fill an empty space before they pair up. Since there are only
3 empty spaces but 5 p electrons, four of the electrons must double
up. The Lewis Dot Diagram should look like:
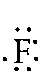
Neon: 1s2 2s2
2p6 -- 2 is the outer
energy level. Since it is 2s2 and 2p6,
there are 8 electrons in the outer energy level. Remember, both of the
electrons in the s-orbital have to be written together. The 6 p-orbital
electrons will each fill an empty space before they pair up. Since there
are only 3 empty spaces but 6 p electrons, all six of the electrons
must double up. The Lewis Dot Diagram should look like: